

Di-Tert-Butyl Peroxide (DTBP) may not be a household name, but it plays a crucial role in a wide range of industrial applications. From polymerization to the production of resins, this versatile chemical is used as a powerful initiator in various chemical reactions. Whether you’re involved in manufacturing plastics, working with adhesives, or exploring chemical synthesis, understanding how DTBP is made and its key applications can significantly benefit your operations. In this article, we’ll walk you through what Di-Tert-Butyl Peroxide is, how it’s made, and why it’s indispensable in many industries today.

Di-Tert-Butyl Peroxide (DTBP) is a chemical compound commonly used as an initiator in polymerization reactions, especially in the production of plastics and resins. It is also used in the synthesis of various organic chemicals. DTBP is known for its stability and efficiency in initiating free-radical reactions, making it a vital tool in the chemical and manufacturing industries.
Di-Tert-Butyl Peroxide plays a key role in industrial applications due to its ability to break down into free radicals. This characteristic is what makes it essential in the production of polymers, resins, and other chemical products. In addition, DTBP is used in the manufacture of coatings, adhesives, and some pharmaceuticals.
DTBP is typically produced through a process known as the thermal decomposition of tert-butyl hydroperoxide. The reaction involves heating the hydroperoxide, causing it to break down into two molecules of Di-Tert-Butyl Peroxide. This method is highly effective, although careful temperature control is necessary to prevent unwanted side reactions.
The main chemical reaction that takes place is as follows in the reaction process:
(CH3) 3COH+H2SO4→(CH3)3COHSO3+H2O
(CH3)3COHSO3+ H2O2→ (CH3)3COOH (tertbutyl peroxide)+H2SO4
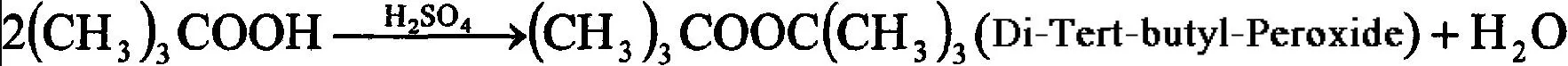
While the process of making Di-Tert-Butyl Peroxide is straightforward, there are a few critical factors to consider:
Temperature Control: The reaction must be carefully controlled to avoid over-heating, which could lead to decomposition or dangerous reactions.
Purity: Purifying Di-Tert-Butyl Peroxide after synthesis ensures its effectiveness as a polymerization initiator.
Safety: Proper handling and storage of Di-Tert-Butyl Peroxide are essential, as it is a reactive substance.
Once produced, Di-Tert-Butyl Peroxide has a variety of industrial uses, including:
Polymerization initiator for plastics and resins
Key ingredient in adhesives and coatings
Used in chemical synthesis to produce other organic compounds
Potential applications in the pharmaceutical industry
As with any reactive chemical, Di-Tert-Butyl Peroxide requires safe handling practices. It should be stored in cool, dry, and well-ventilated areas, away from heat sources or ignition points. Protective gear such as gloves and goggles should be worn during handling to avoid exposure.
If you're in search of a trusted DTBP(Di-Tert-Butyl Peroxide) manufacturer that delivers top-quality products and dependable service, look no further than High Mountain. With years of experience in producing high-grade DTBP, High Mountain stands out for its commitment to excellence, strict quality control, and adherence to industry standards. Whether you need bulk quantities or custom solutions, our team is ready to meet your specific requirements. Contact High Mountain today and ensure your business has the reliable DTBP supply it needs to succeed.

Di-Tert-Butyl Peroxide is an essential compound in many industrial applications, particularly in polymerization and chemical synthesis. Understanding the process of making DTBP, along with the safety measures required, can help ensure its effective and safe use in various industries. Whether you are involved in chemical manufacturing, polymers, or adhesives, Di-Tert-Butyl Peroxide is a chemical you should be familiar with.









