

Tert-Butyl Benzoate Peroxide (TBPB) is a common chemical compound in various industrial applications. If you’re wondering about its solubility in water, you’re not alone. This article provides an in-depth look into TBPB, exploring whether it’s soluble in water and what factors influence its solubility.
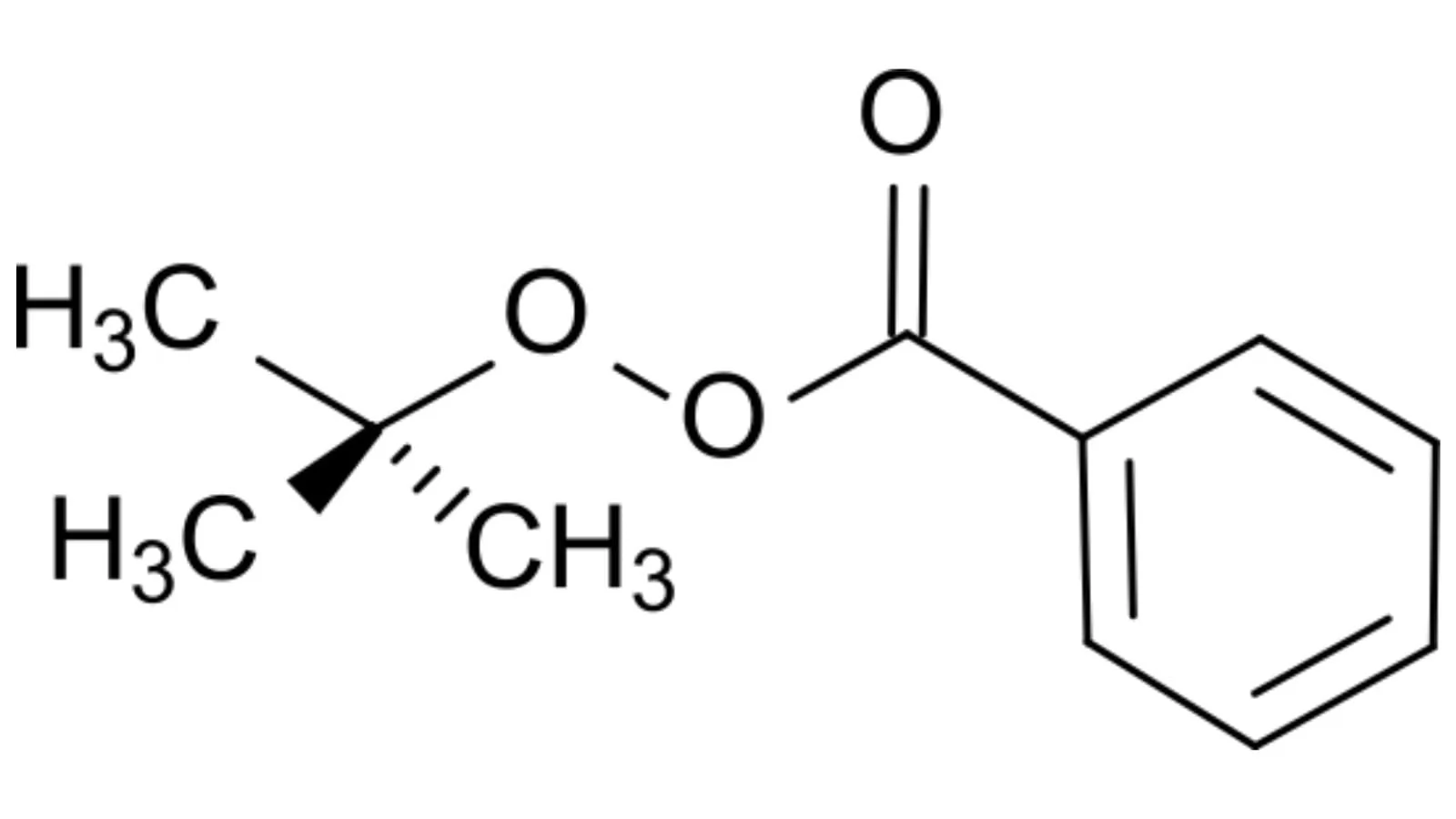
Tert-Butyl Benzoate Peroxide, often abbreviated as TBPB, is an organic peroxide that functions as a catalyst or initiator in polymerization processes. TBPB is valued for its stability under standard conditions and plays a significant role in producing polymers, resins, and other industrial products.
Understanding why some substances dissolve in water while others don’t requires a basic grasp of solubility principles. Essentially, solubility depends on the interaction between molecules of a substance and the solvent—in this case, water. Water is a polar solvent, which means it dissolves polar substances easily. However, nonpolar substances tend not to dissolve well in water.
TBPB is generally considered to be poorly soluble in water. As a compound with a nonpolar structure, TBPB does not interact well with water molecules, which are highly polar. This lack of interaction is why TBPB doesn’t dissolve easily in water, making it hydrophobic (water-repellent).
Several factors impact TBPB's solubility, particularly when it comes to its interaction with water. Here are some of the primary factors:
If you need to dissolve TBPB, using an alternative solvent may be more effective. TBPB is soluble in various organic solvents, including:
These solvents are nonpolar or less polar than water, allowing TBPB to dissolve much more effectively.
The limited solubility of TBPB in water is actually an advantage in many industrial applications. In polymerization processes, for example, water-insoluble initiators like TBPB provide controlled reactivity, enhancing the process's stability. Its stability in organic solvents also makes TBPB highly adaptable for various manufacturing needs.
Due to its reactive nature, TBPB should be handled with care, especially in concentrated forms. Follow these guidelines for safe handling:
Choosing High Mountain Chem as your manufacturer for Tert Butyl Benzoate Peroxide guarantees access to high-quality products, competitive pricing, and dedicated support. Their expertise in the chemical industry, coupled with a commitment to excellence, positions them as a leading choice for businesses looking to enhance their product offerings and operational efficiency.
In summary, Tert-Butyl Benzoate Peroxide is not soluble in water, primarily due to its nonpolar structure. If you need it dissolved, consider using an organic solvent like acetone or toluene. TBPB’s properties make it valuable in industrial applications where water resistance is beneficial, such as in controlled polymerization processes.
Understanding the solubility characteristics of TBPB can help ensure its safe and effective use across various applications. Remember to always prioritize safety when handling and storing this compound.








