

Dive into the multifaceted realm of Sodium Formaldehyde Sulfoxylate (SFS), a paramount chemical compound that underpins various industrial applications. From its chemical composition, production process, quality control, to its widespread use in textiles, food preservation, and medicine, explore how SFS is an indispensable part of modern manufacturing.
Sodium Formaldehyde Sulfoxylate, abbreviated as SFS, is a versatile chemical compound that plays a significant role in various industrial applications. Its chemical formula is NaHSO2·CH2O2, showcasing a combination of sodium, sulfur, hydrogen, carbon, and oxygen atoms. This compound is well-regarded for its reductive properties, making it a go-to solution in many reduction processes across different industries.
The manufacturing sector, particularly, benefits from the reductive capabilities of Sodium Formaldehyde Sulfoxylate. It's deployed as a bleaching agent in the textile industry, helping to eliminate unwanted pigments from fabrics, thus ensuring the desired color quality is achieved. By breaking down the dye molecules, SFS aids in attaining a cleaner, more vibrant look for the fabrics.
Moreover, Sodium Formaldehyde Sulfoxylate acts as a potent antioxidant. In the realm of polymer production, it's used to prevent the degradation of materials by halting the oxidative processes that could potentially harm the integrity and quality of the products. By doing so, it extends the lifespan and maintains the aesthetic appeal of polymer-based items.
In the realm of food processing, Sodium Formaldehyde Sulfoxylate is employed as a preservative. It aids in maintaining the freshness and extending the shelf-life of various food products by thwarting the growth of microbes which could lead to spoilage.
Additionally, in the photographic industry, SFS serves as a developer reducing agent. It facilitates the processing of photographic films and papers, aiding in the revelation of images captured. Its reductive nature enhances the clarity and quality of photographs, making it an essential asset in photographic processing.
The multifaceted utility of Sodium Formaldehyde Sulfoxylate underscores its importance in contemporary industrial applications. Its ability to act as a reducing agent, an antioxidant, and a preservative makes it a valuable commodity for manufacturers striving to uphold quality and efficiency in their operations.
The ubiquitous presence of Sodium Formaldehyde Sulfoxylate (SFS) in everyday items is a testimony to its significance in modern-day manufacturing and consumer goods. Its unique properties make it a staple in a plethora of products that we come into contact with on a daily basis.
In the textile industry, the clothes we wear often undergo a bleaching process with the help of SFS to attain the desired hue and to remove any impurities that may affect the fabric's appearance. The bright and clean appearance of textiles is often achieved through the reducing and bleaching actions of Sodium Formaldehyde Sulfoxylate.
Moving over to our kitchens, the preservation of certain food products is made possible through the use of SFS. It acts as a preservative, keeping food fresh for longer and ensuring the safety and quality of food products on the shelves. This not only extends the shelf-life of food items but also reduces the risk of foodborne illnesses.
Our photographic memories, too, owe a nod to Sodium Formaldehyde Sulfoxylate. The chemical aids in the development of photographic films and papers, bringing our captured moments to life with clarity and precision. Its role as a reducing agent in the developer solution helps in achieving the desired contrast and quality in photographs.
Furthermore, in the realm of polymer manufacturing, products made of plastic and other polymer materials benefit from the antioxidant properties of SFS. It prevents the degradation of materials, ensuring the durability and longevity of a wide range of consumer goods, from household appliances to automotive parts.
The presence of Sodium Formaldehyde Sulfoxylate extends to the healthcare sector as well. Its antioxidant properties find applications in certain pharmaceutical formulations, contributing to the efficacy and stability of some medicines.
Sodium Formaldehyde Sulfoxylate (SFS) is a fascinating compound with a unique chemical composition that lends itself to a variety of applications. Understanding its chemical structure is crucial for appreciating its functionality and utility across various industries.
The chemical formula of Sodium Formaldehyde Sulfoxylate is NaHSO2·CH2O2. This formula represents a compound comprised of sodium (Na), sulfur (S), oxygen (O), hydrogen (H), and carbon (C) atoms. The structural formula reveals a sodium atom linked to a sulfoxylate ion, which itself is a combination of sulfur dioxide and formaldehyde molecules. This arrangement of atoms and molecules characterizes the reducing capabilities and other properties inherent to SFS.
When compared to similar compounds, Sodium Formaldehyde Sulfoxylate stands out for its reductive and antioxidant properties. For instance, Sodium Bisulfite (NaHSO3) is another reductive agent used in various applications, including food and beverage processing. However, SFS tends to have a stronger reducing potential and is more stable under a broader range of conditions, making it a preferred choice in many industrial applications.
Another comparable compound is Sodium Sulfite (Na2SO3), which also finds usage as a reducing and preserving agent in different sectors. Yet, the reductive strength and antioxidant capacity of Sodium Formaldehyde Sulfoxylate often surpass those of Sodium Sulfite, making SFS a more effective and versatile choice.
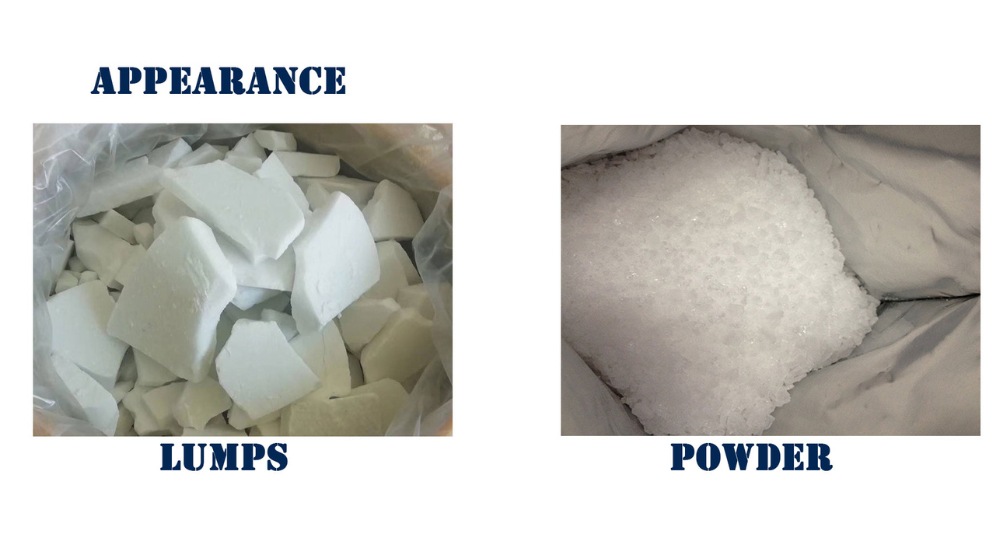
The behavior of Sodium Formaldehyde Sulfoxylate (SFS) in different conditions and its interaction with other substances is a topic of paramount importance for industries that utilize this compound. The reactivity and stability of SFS significantly influence its effectiveness and safety in various applications.
Sodium Formaldehyde Sulfoxylate is renowned for its reducing capabilities. When introduced to oxidizing agents, it undergoes redox reactions where it acts as a reducing agent. In these reactions, SFS donates electrons to the oxidizing substances, undergoing a transformation that aids in the reduction of other compounds while becoming oxidized itself.
The stability of Sodium Formaldehyde Sulfoxylate is influenced by various factors, including temperature, pH levels, and exposure to other chemicals. Here are some insights into how these factors affect the stability of SFS:
The production of Sodium Formaldehyde Sulfoxylate (SFS) is a meticulous process that involves specific raw materials and a defined synthesis procedure. Ensuring a high level of precision and adherence to the established steps is crucial for obtaining SFS of the desired quality and purity.
The primary raw materials required for the synthesis of Sodium Formaldehyde Sulfoxylate are sodium hydroxide (NaOH) and formaldehyde (HCHO). Sodium hydroxide is usually sourced from the electrolysis of sodium chloride, while formaldehyde can be produced through the oxidation of methanol. The sourcing of these materials involves engaging with suppliers who adhere to strict quality and environmental standards to ensure the purity and reliability of the raw materials.
The synthesis of Sodium Formaldehyde Sulfoxylate encompasses the following steps:
Quality control is an indispensable aspect of the production of Sodium Formaldehyde Sulfoxylate (SFS), ensuring that the final product meets the requisite standards and specifications. A stringent quality control process ensures that each batch of SFS is consistent in quality, thereby fulfilling the expectations of various industries that rely on this compound.
Testing is a pivotal part of the quality control process. Various parameters such as purity, pH, reductive strength, and the absence of impurities are tested to ensure they meet the specified standards. The testing procedures may include titration methods, spectroscopy, chromatography, among others, to ascertain the quality of SFS. Adhering to internationally recognized standards like those set by the American Chemical Society (ACS) or the International Organization for Standardization (ISO) is crucial to ensure the quality and safety of the produced SFS.
Achieving consistency across different batches of SFS is a hallmark of a robust quality control system. Here’s how consistency is maintained:
Sodium Formaldehyde Sulfoxylate (SFS) has carved a niche for itself across a spectrum of industries due to its distinctive chemical properties. Its versatility as a reducing agent and an antioxidant opens up a plethora of applications, contributing to enhanced processes and better products. Here's a dive into the common applications of SFS in various sectors:
The textile and leather industries extensively utilize SFS as a bleaching and reducing agent. It helps in the removal of unwanted pigments, ensuring the desired color and quality of fabrics and leather products. Additionally, SFS aids in processing and finishing operations, enhancing the texture and appearance of the final products.
In the food industry, SFS acts as a preservative, extending the shelf-life of various food products by inhibiting microbial growth. Its antioxidant properties also help in maintaining the freshness and quality of food by preventing oxidation, a common cause of spoilage and flavor degradation.
Apart from mainstream industries, SFS finds applications in niche areas such as photographic processing where it serves as a reducing agent, aiding in the development of photographic films and papers. Its ability to facilitate redox reactions makes it a valuable component in the photographic development process.
The antioxidant properties of SFS are harnessed in the pharmaceutical sector to enhance the stability and efficacy of certain medications. By inhibiting oxidative degradation, SFS contributes to the preservation of the medicinal properties and extends the shelf-life of pharmaceutical products.
The reducing capability of SFS is leveraged across various industries including polymer production, water treatment, and metal processing. By donating electrons, SFS aids in reducing other substances, facilitating reactions that are crucial for the desired outcomes in these industries. Its role as a reducing agent is fundamental in driving processes and achieving quality products across a wide array of industrial applications.
In the extensive domain of chemical compounds, Sodium Formaldehyde Sulfoxylate (SFS) emerges as a cornerstone, driving advancements in numerous industries. Its unique properties and applications make it a staple for manufacturers aiming for superior quality and efficacy in their products. Among the frontrunners in supplying high-grade SFS is High Mountain Chem, a reputable manufacturer synonymous with quality and reliability. Explore their offerings and learn more about how they are fueling industries with top-notch SFS at www.highmountainco.com. The journey of discovering the potential of SFS unravels endless possibilities, paving the way for enhanced processes and superior products in a myriad of applications.









